How are vaccines developed, and how do we know they are safe?
Vaccine development is regulated and monitored at every stage. A vaccine is only approved if it is deemed safe and effective, following a rigorous assessment of its benefits and risks.
Those Nerdy Girls is your trusted source for all things health. Whether there’s too much information, not enough information, or lots of misinformation, we have you covered. Help us keep the lights on by becoming a paid subscriber today.
How are vaccines developed, and how do we know they are safe?
Vaccine development is regulated and monitored at every stage. A vaccine is only approved if it is deemed safe and effective, following a rigorous assessment of its benefits and risks. This ensures that you can trust vaccines offered by your healthcare professional, even those developed during emergencies like the COVID-19 pandemic.
Vaccines have saved A LOT of lives. A recent study estimated that vaccines have prevented 154 million deaths globally since the mid 70s (equivalent to 40% of the US population today).
But what’s the process for developing vaccines? Let's start at the beginning and break it down!
A little history
Luckily the days of physicians such as the British doctor Edward Jenner jabbing 8-year-olds (!) with ground up tissue from the scab of a milkmaid suffering from cowpox are long gone.This is how in 1796, Jenner discovered that this method provided the young boy with immunity from smallpox, a related virus that was raging through the population - a bit of a gamble and not ethical by today’s standards, but it paid off! And this is essentially how the idea of vaccines and vaccination was born. It is important to note that before Jenner more formally “invented” vaccines, this technique called variolation had been practiced for many years in the Far East and an enslaved West African man, named Onesimus by his Bostonian owner, brought this to the US in 1716.
(Fun fact - The word vaccine comes from the Latin word for cow “vacca”)
Fast forward to 1902 and the first federal regulation for vaccines emerged in the US in the form of the Biologics Control Act.
Later the United States Public Service Act of 1944 mandated that the federal government issue licenses for biological products, including vaccines.
And in 1954 the Division of Biologics Standards (now part of the FDA) was formed to oversee vaccine safety and regulation.
Today, the U.S. Food and Drug Administration (FDA) continues to be the regulatory authority which oversees the safety, effectiveness and quality of vaccines that are developed and available in the US.
But how are vaccines developed, and what safeguards are in place to make sure the vaccines available to us are safe?
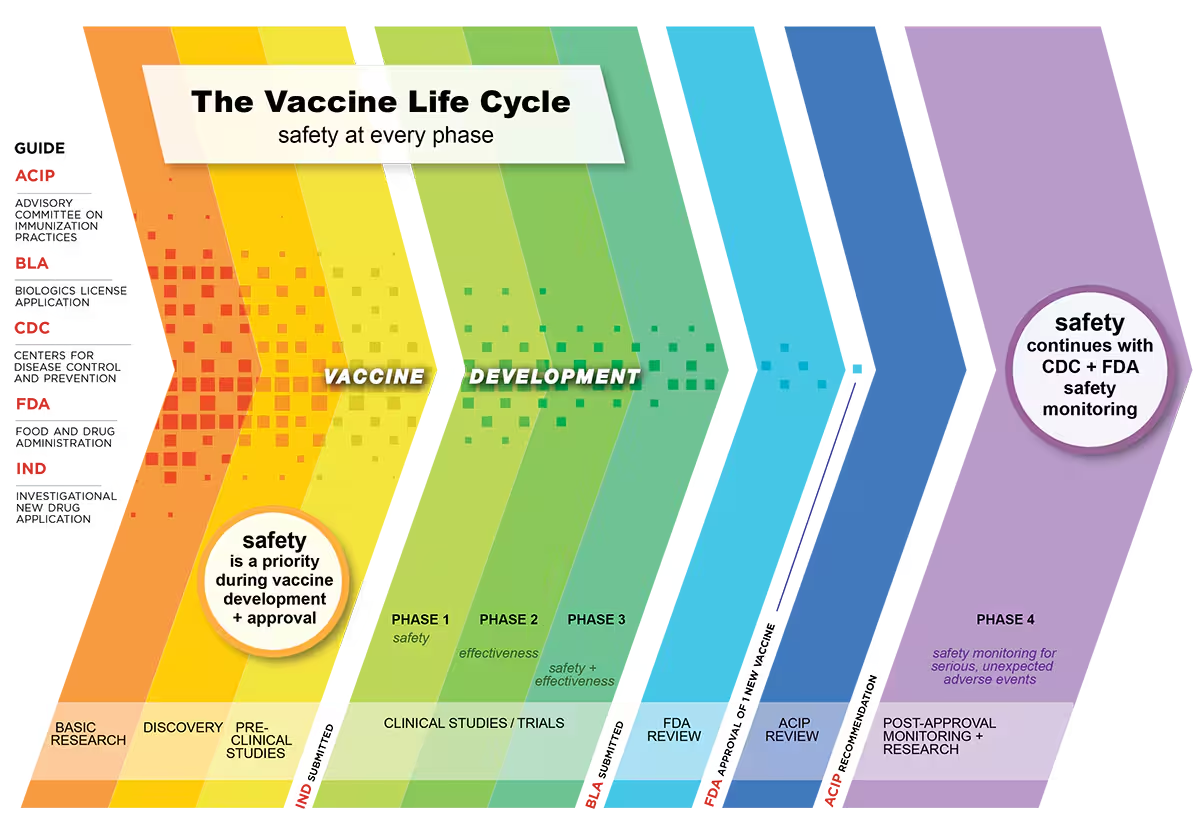
Phases of vaccine development
Source: CDC
🔬 R&D - Research and Development
In this first stage scientists determine for which disease a vaccine should be developed. This depends on how infectious a disease is, how it spreads and what kind of risk it poses to the public.
🧬 Pre-Clinical trials
These are always animal studies to find out if the vaccine is safe and evokes the desired immune response to the disease being studied. Once this has been completed an application to the FDA can be made to proceed to human trials. This is called an Investigational New Drug (IND) application.
🩺 Clinical development - This is split into three phases.
Phase 1 - In this first phase the vaccine is given to a small number of healthy volunteers aged 18 and older (under 100 participants) who are very closely monitored. The main aim at this early stage is to find out about safety and if there are any adverse reactions. Any data on whether the vaccine elicits an immune response are also collected.
Phase 2 - If there are no safety concerns from the Phase 1 trials, Phase 2 trials begin and a larger group of participants (usually several 100s) is enrolled. These include people from different backgrounds with a variety of health statuses, some healthy, some with existing health conditions (NOTE - these are all volunteers! ). Apart from looking at safety and side effects, different dosages of the vaccine with different immune responses are also assessed. In addition, first indications of vaccine efficacy (how well a vaccine works in a controlled environment) are also established. In this as in all phases there are very strict protocols with standardized tests to ensure the results are repeatable and the data are closely monitored and scrutinized throughout.
Phase 3 - This is the last stage before the vaccine developer can apply for approval. During Phase 3 trials 1000s of participants are followed for at least 2 months to identify any potential side effects. This is usually carried out in efficacy trials where some participants are given the vaccine and others a placebo. Data are collected on many aspects and safety continues to be monitored throughout, as well as information on how effective the vaccine is. As more people are in this trial, often additional information on side effects that are less common can also be collected.
📢 If at any stage an issue with safety is detected, trials are halted and a vaccine will not go through to the licensing stage until the concerns have been addressed!
🧾 Biologic and License Application (BLA)
Following the successful completion of the clinical trial phases a company can then submit a “BLA”. This application asks for “permission to distribute and market a vaccine for use in the United States”. The FDA looks at all the safety and efficacy data from the clinical trials as well as information on how the company plans to ensure ongoing quality and consistency of the vaccine.
✅ Approval by FDA
The FDA takes many factors into consideration before approving a vaccine for production and roll-out to the population. It has a whole team of scientists, statisticians, safety experts and manufacturing specialists who work together to analyze the risks and benefits to the vaccine’s intended population..
✅ Recommendation by the CDC’s Advisory Committee on Immunization Practices (ACIP)
Following FDA approval the U.S. Centers for Disease Control and Prevention’s ACIP then develops recommendations on how to use the vaccines.
This is a group made up of 15 medical and public health experts who are selected by the Secretary of the Department of Health and Human Services (DHHS). Their expertise lies in the fields of vaccinology, immunology, pediatrics (child health), internal medicine, nursing, family medicine, virology, public health infectious diseases and preventive medicine. In addition one person comes from the public to provide a view on social and community aspects.
ACIP makes its recommendations based on:
How safe and effective a vaccine is for different age groups
How serious the disease for which the vaccine was developed is, i.e. can it potentially cause long-term problems (e.g Long Covid) or death
How many would get the disease if they were not vaccinated - this is looking at the public health benefit
So finally…
JABS IN ARMS 💪🏻💉💪💉💪🏼💉💪🏽💉💪🏾💉💪🏿💉
The vaccine is rolled out to the general population (or groups of the population e.g. different age groups or those who are most vulnerable).
🔎 Surveillance after approval
Even after approval by the FDA and roll-out, vaccines continue to be monitored for safety and effectiveness. These are sometimes called Phase 4 development studies. These post-marketing safety studies, involving hundreds of thousands of participants, successfully identified rare side effects of the J&J and AstraZeneca vaccines.
In addition there are several reporting systems that the public can access to report any side effects.
Here are some examples:
VAERS (Vaccine Adverse Event Reporting System) is one of the surveillance systems that is managed by both the FDA and CDC. Anyone can report an adverse event after they have received a vaccine through their online portal or by filling out and posting a form. Although this information cannot establish if there is a clear link, it is used to identify if there are common occurrences that might need further investigation, which might need for the vaccine to be paused. As anyone is able to report to this system, it is unfortunately vulnerable to misuse and anti-vaccination groups exploit this weakness..
During the Covid-19 pandemic the CDC developed the smartphone app V-Safe to help track side effects. This is still in operation today and has been expanded to also cover RSV vaccines.
But if there is a global emergency, like the Covid-19 pandemic, don’t regulators make shortcuts to speed up the process?
It is true that Covid vaccines were developed and approved faster than previous vaccines, however this does not mean that shortcuts were made.
There is a process called EUA (Emergency Use Authorization) which ensures that even in a public health emergency a rigorous scientific and regulatory process is followed to ensure maximum safety. The decision to authorize a vaccine under these circumstances is based on weighing up potential benefits of a vaccine against the risk to the public, especially in the absence of available treatment or already authorized alternatives. In a situation like a pandemic, the FDA can use EUA to expedite the approval process which is based on the best available evidence from existing trials. In the case of the Covid vaccines, these were preliminary data from thousands of participants in Phase 3 clinical trials who had been followed for two months before the EUA was issued (remember, Phase 3 is the final step, so a lot of assessment for safety and efficacy has already been undertaken)
What happens in the rest of the world?
🇪🇺 The European Union has a similar process to the US and the European Medicines Agency supervises and regulates all vaccines.
🇬🇧 In the United Kingdom the governing body is called MHRA (Medicines and Healthcare Products Regulatory Agency).
🌍 Many poorer countries who do not have their own regulatory bodies will defer to FDA or EMA recommendations, and the World Health Organization also provides standards and recommendations for the regulation of vaccines.
Bottom line
You can feel confident that vaccines that come to the market have gone through a rigorous process to ensure they are safe and do their job. And after initial approval, close monitoring continues and anyone who has received a vaccine can report any side effects or adverse events.
In an emergency, the approval process is sped up but no shortcuts are made.
Stay safe, stay well, and stay vaccinated
Love,
Those Nerdy Girls
Further resources:
History:
Vaccine Development, Testing, and Regulation
Vaccine development 101:
CDC - How Vaccines are Developed and Approved for Use
Children’s vaccine journey:
CDC - The Journey of Your Child’s Vaccine
Vaccines save lives:
ACIP:
EUA:
FDA - Emergency Use Authorization for Vaccines Explained
More TNG Posts on Covid vaccine development, EUA and how side effects are tracked:
Were COVID-19 vaccines developed too fast to be safe?
How are possible side effects from COVID-19 vaccines evaluated and tracked?
In vaccine trial data we trust?!
Reminder that we now have an online store filled with amazing nerdy merchandise.
Your purchases will help financially support the science communication mission of Those Nerdy Girls.
P.S. We’d love to see pics of you and your friends and family sporting their new TNG swag. You might even be featured in our shop! Email us your pics at info@dearpandemic.org
Like what you read? Please share it with others!
If you have a question, let us know!
We read every question and use them to inform our upcoming content, though we are unable to respond to each specific question.